|
Poster10: 3-Me-1-H- Indazole and Fluoride Sequestration in SNAr Reactions in Flow
|
|
|
|
Poster 9: Accessing Amides Quickly, Cleanly and on Multi-Gram Scales with DABAL-Me3
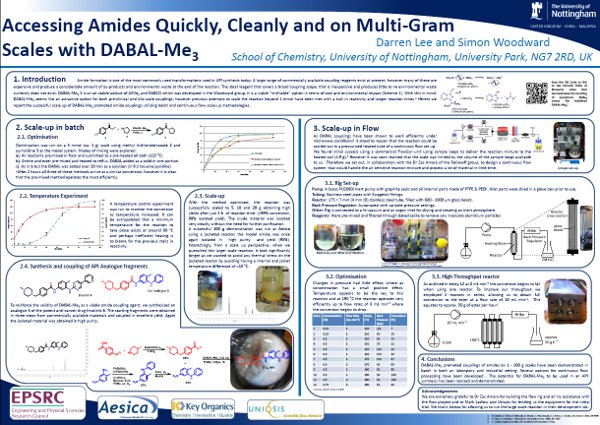
Methods for the batch scale up of DABAL-Me3 promoted direct ester to amide synthesis have been demonstrated at 10–100 g scales using a tert-amide model compound. A Continuous flow methodology provides a method for larger scales (productivities of >50 g h–1). In addition, nitriles were coupled to primary amines and hydrazines with DABAL-Me3, resulting in the clean formation of free amidines (16 examples) and amidrazones.
|
|
|
|
Poster 8: Continuous Flow N-arylation of Aniline Derivatives

A continuous flow strategy has been applied to the N-arylation of anilines using diaryliodonium salts.
This approach has been demonstrated to be directly scalable, without the need for further reaction optimisation. When utilising a copper coil reactor it is possible to effect this transformation in high yield at room temperature for selected examples.
Mesitylene has been shown to be a non-participating ligand for the metal catalysed arylation, thereby facilitating efficient selective arylation to be performed when desired.
|
|
|
|
Poster 7: Ireland-Claisen Rearrangements in a Continuous Flow Process

The Ireland-Claisen Rearrangement is a well-known and powerful tool in organic synthesis. It enables the formation of gamma, delta-unsaturated acids from their corresponding allylic esters by [3,3] sigmatropic rearrangement. Typically the allylic ester is subjected to a strong base to form the enolate ester, which can then readily rearrange to produce the desired acid. Even though this rearrangement was an improvement from previous Claisen reactions (harsh conditions, low yields), it still employed long reaction times and reagents that required special handling, such as alkyl lithiums and air-sensitive catalysts.
To overcome these obstacles, we have utilized continuous flow microreactors to aid in lowering reaction times and minimize handling of reagents.
Initial results have shown reduced reaction times for the standard rearrangement conditions.
|
|
|
|
Poster 6. Flow Synthesis of Fanetizole using Gaseous Ammonia in a Teflon AF-2400 'Tube-In-Tube' Reactor
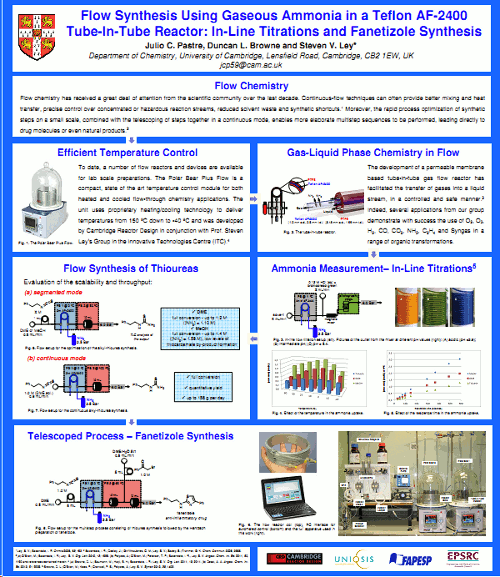
The recent development of 'tube-in-tube' gas flow reactors employing a permeable membrane (Teflon AF-2400) has facilitated the safe and controlled introduction of gases into flowing liquid streams. The Ley group has exemplified this strategy using a variety of reactive gases to perform organic synthesis under continuous flow-through conditions: e.g.O2, O3, H2, CO, CO2, NH3, C2H4;and Syngas (H2:CO 1:1).
In particular, 'tube-in-tube' gas-liquid reactors can be utilised to produce homogeneous reaction streams that contain a known quantity of a gas, thereby enabling stoichiometric gas-liquid reactions to be performed with reproducible residence times.In the present study using ammonia gas, a simple in-line titration technique was used to quantify the concentration of gas obtained in a selection of solvents at different temperatures and for different residence times.
This information was then used to prepare a thiourea which was then scaled and telescoped with a Hantsch thiazole synthesis to afford a continuous flow-through synthesis of the anti-inflammatory agent fanetizole at a rate of approximately 10 g/h.
|
|
|
|
Poster 5: Iodination and Protection of Heterocycles in Flow
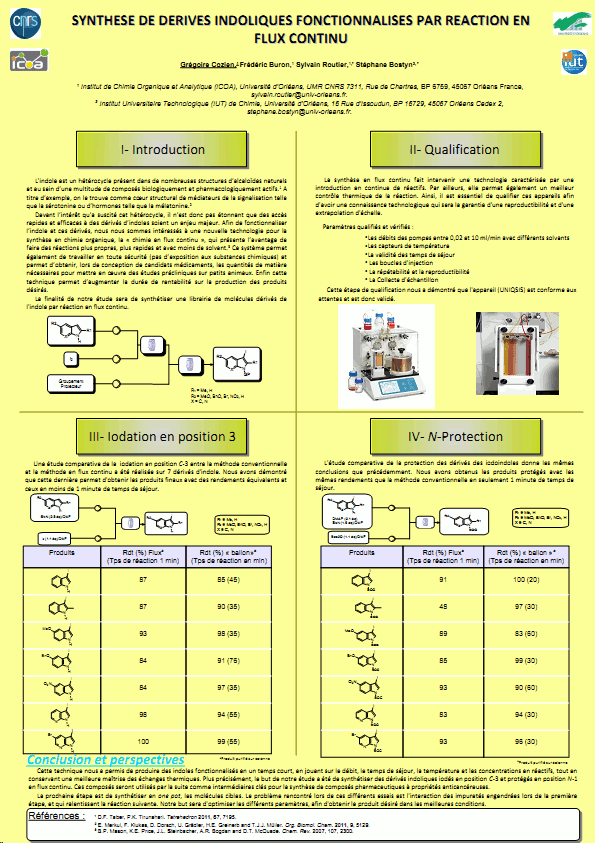
Researchers at the University of Orleans report a 2 stage, continuous flow-through approach for the iodination of indoles and related heterocycles followed by their subsequent BOC protection.
Yields and purities reported contrast favourably with those obtained under batch conditions, however much shorter reaction times were achievable in flow.
Indoles and related heterocycles are important chemotypes of considerable interest in drug discovery.
|
|
|
|
Poster 4: Control of Mixing and Temperature using Static Mixers

Meso-scale Glass Static Mixers can be used as scaleable flow reactors for rapid chemistries. This poster was first presented at the Uniqsis Flow Chemistry Symposium, Cambridge, 2009.
|
|
|
|
Poster 3 (Sygnature): Preparation of Azides under Segmented Flow Conditions
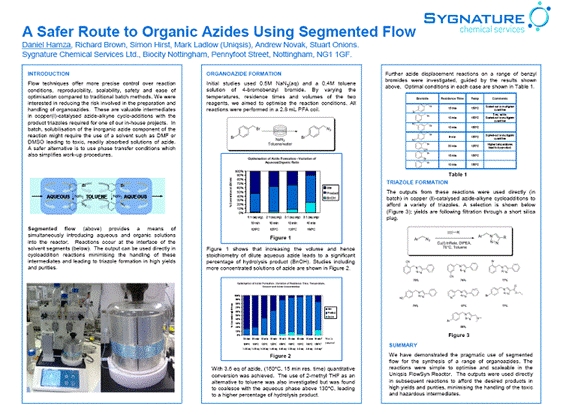
Azides can be prepared by nucleophilic displacement of halides under segmented flow-through conditions in a flow reactor. This poster was first presented at the Uniqsis Flow Chemistry Symposium, Cambridge, 2009.
|
|
|
|
Poster 2: FlowSyn: An Integrated Bench-top Continuous Flow Reactor

Please download the poster which was presented at the Pittcon exhibition/conference 2008 in New Orleans.
|
|
|
|
Poster 1: Flow Synthesis: Reaction Optimisation and Scale-up in Meso-reactors
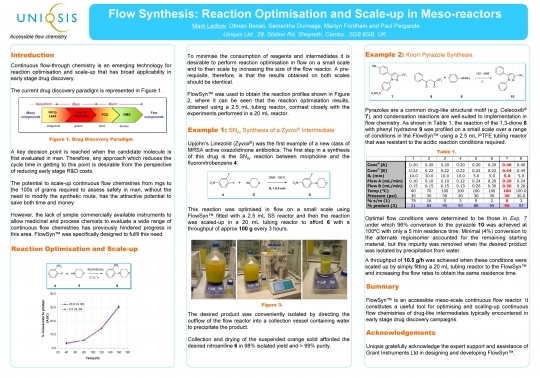
Please download the poster which was presented at the Pittcon exhibition/conference 2008 in New Orleans.
|