|
Note 34: Photochemical Quinoline Synthesis and Minisci Functionalisation
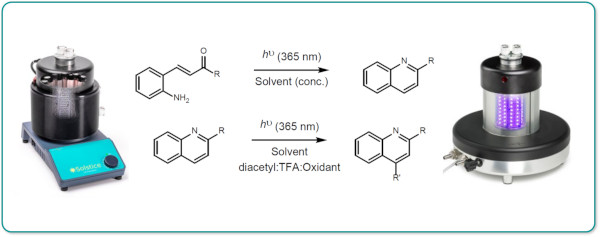
Quinoline formation and subsequent Minisci functionalisation under photochemical conditions with irradiation at 365nm were investigated and optimised under small-scale batch conditions using the Uniqsis Solstice 12-position photoreactor.
The optimised conditions were utilised for scale-up under continuous flow conditions using the Borealis Flow reactor.
Notably the space-time yield was determined to be considerably higher in flow than batch.
|
|
|
|
Note 33: Accelerated Metal-mediated Photocarboxylative Arylation
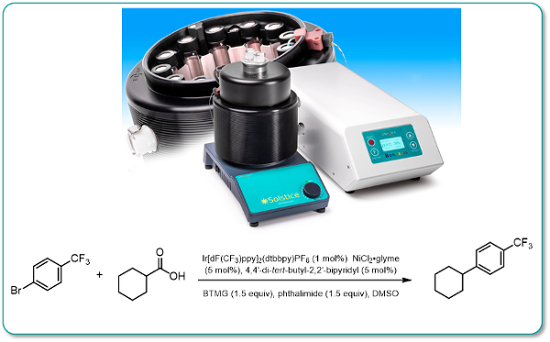
An upgraded high-output 420nm Borealis LED lamp was compared to the standard version and shown to give a significant rate enhancement in a representative metal-mediated photodecarboxylative arylation reaction.
The new high-output Mk2 Borealis lamps are backward compatible with existing Borealis digital power supplies.
|
|
|
|
Note 32: A 2-Step, Continuous Flow Photochemical and Thermal Cascade
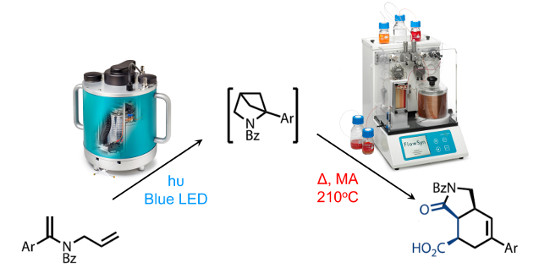
A triplet sensitised, excited state photochemical [2+2] ring closure was successfully combined with a subsequent high temperature thermal electrocyclic cascade under continuous flow-through conditions to afford bicyclic lactams in high overall yield and excellent purity.
This process was facilitated by the ability to employ MeCN as solvent at high temperature under pressurised conditions and the precise control of residence time and temperature in the flow reactor enabled the isolation of a reactive diene intermediate on a scale and yield not achievable under batch conditions.
|
|
|
|
Note 31: Efficient Ring-Closing Metathesis under Flow-Through Conditions
.jpg)
Olefin metathesis is an important reaction that provides a useful route to highly functionalised molecules such as drug precursors or polymers. A recent report from Dr. Lamaty's group in Montpellier illustrates a scalable flow-through adaption of this reaction.Although this reaction is highly efficient in batch, scaling-up usually induces poor reproducibility. Thus, several examples have been reported for the development of olefin metathesis in continuous flow, most of them focus on the use of heterogeneous catalyst, that, for the moment, show limited recyclability. The use of a homogeneous catalyst in continuous flow, and especially in an environmentally friendly solvent, brings this reaction on step further toward scale-up at the industrial level. This reaction system is well suited for continuous flow implementation as short reaction times, elevated temperatures and high conversion can be achieved while using dimethyl carbonate as a solvent.
|
|
|
|
Note 30: Copper Catalysed N-Arylation in Flow
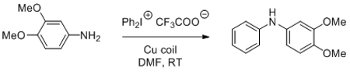
The diarylamine functionality is an important chemotype in medicinal chemistry and also occurs widely in nature.
Its synthesis typically involves Ullman-type couplings in the presence of metal catalysts (e.g. Cu, Pd). However, the synthesis conditions are often harsh, require extended reaction times and may give low yields.
Diaryliodonium salts are effective alkylating for a range of substrates including anilines. In this Application Note, researchers at Newcastle University show that this reaction is effectively catalysed by using a copper coil reactor under flow-through conditions such that the reaction occurs at room temperature. Moreover, only moderate reaction times are required to give high yields.
|
|
|
|
Note 29: Pyrazole Formation using a 2 Step Reaction in Flow
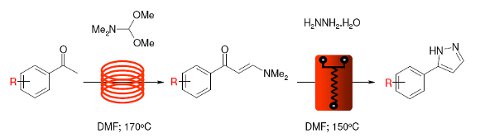
Pyrazoles are an important and well represented chemotype in drug discovery displaying wide-ranging biological actions that include analgesic, anti-inflammatory, antipyretic, tranquilizing,muscle relaxing,anticonvulsant, antidiabetic and antibacterial activities. The Pfizer Cox-2 inhibitor Celecoxib is a notable recent example.
This Application Note describes an efficient 2 step synthesis of a library of pyrazoles under continuous flow-through conditions developed by chemists at GalChimia. A starting acetophenone is first condensed with DMFDMA to form an intermediate enaminone followed by a second condensation with hydrazine to generate the desired pyrazoles.
The final condensation is performed using a Uniqsis glass static mixer chip.
|
|
|
|
Note 28: Amide Bond Formation in Flow
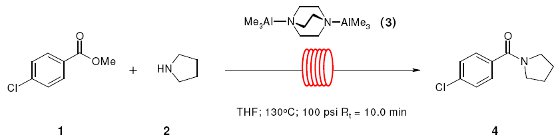
The formation of amide bonds from esters and carboxylic acids is an important reaction in organic synthesis.
Whilst this can be achieved in the presence of trimethylaluminium (TMA), this reagent is a pyrophoric gas at room temperature and is both hazardous and inconvenient to use. The complex between trimethylaluminium and DABCO (DABAL-Me3) is a white solid that is more readily handled and therefore represents an attractive alternative.
Amide bond formation occurs within a few minutes at elevated temperatures under flow-through conditions.
|
|
|
|
Note 27: Flow Nitration of Electron Deficient Aromatics
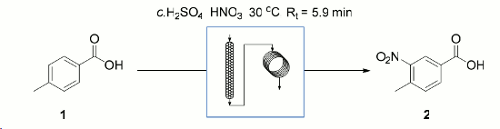
Nitration is an extremely useful reaction for the functionalisation of aromatic ring systems.
Due to the exothermic nature of the reaction,especially on large scale, aromatic nitration has the potential to be very dangerous, especially when generating explosive nitro-species. Translation of batch methods to flow chemistry has shown that the fine control over reagent mixing, control of residence time in the reactor and very efficient heat transfer is a much safer method of conducting this type of chemistry.
|
|
|
|
Note 26: Vanillic Acid Esterification
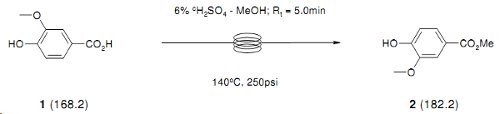
Simple acid catalysed esterifications can be easily achieved under flow-through conditions.
In this example, methyl vanillate was produced with a throughput of approximately 30g/h and conveniently isolated in high purity by precipitation from water.
|
|
|
|
Note 25: Low Temperature Metalation Ver 2
Metalations are typically performed at very low temperature (often -78C) in batch with slow addition of the organometallic base in order to control the exothermic nature of such reactions. In flow, the improved control of mixing and temperature, will often ensure that similar (or improved) reaction outcomes can be achieved more conveniently at higher temperatures.
(Demonstration version).
|
|
|
|
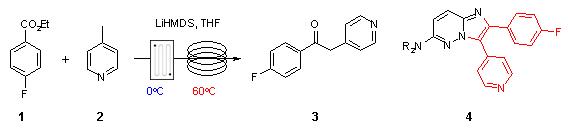
Note 25: Low Temperature Metalation
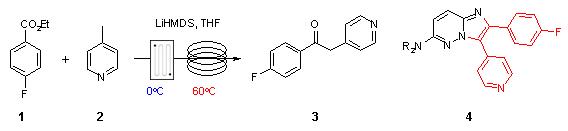
Metalations are typically performed at very low temperature (often -78C) in batch with slow addition of the organometallic base in order to control the exothermic nature of such reactions. In flow, the improved control of mixing and temperature, will often ensure that similar (or improved) reaction outcomes can be achieved more conveniently at higher temperatures
|
|
|
|
Note 24: 3-Hydroxy-4-quinolone
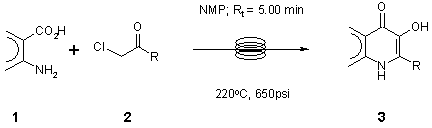
3-Hydroxy-4-quinolones can be prepared in 5 min at 220°C under continuous flow-through conditions. The reaction is readily scalable.
|
|
|
|
Note 23: Benzimidazole Synthesis
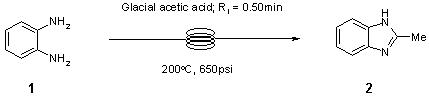
Many useful reactions in chemical synthesis can be considerably accelerated when run at high temperature under microwave-assisted conditions. However, for scale-up the need to empty and refill conventional batch microwave vessels coupled with repetitive heating up and cooling down cycles is both inconvenient and decreases throughput. In this Application Note we describe the continuous flow synthesis of 2-methylbenzimidazole which can be achieved more conveniently in 30 seconds at 200°C under continuous flow-through conditions in the FlowSyn.
|
|
|
|
Note 22: Flow Nitration
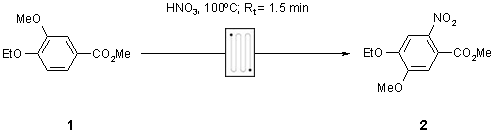
Nitration is an important synthetic procedure that is often difficult to control. Under continuous flow-through conditions, however, exotherms are typically well controlled and nitration can be safely performed on a large scale.
|
|
|
|
Note 21: Bromination
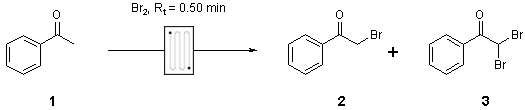
Electrophilic bromination is a rapid and exothermic reaction that can be difficult to control to prevent bis-bromination in batch. Both temperature and mixing can be well controlled using a mixer chip in flow, leading to a highly reproducible outcome.
|
|
|
|
Note 20: Transfer Hydrogenation using Nanoparticular Pd[0]
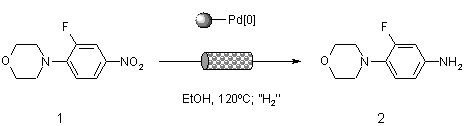
Transfer hydrogenation is a synthetically useful technique that enables hydrogenation to be effected without the need for hydrogen gas. Under superheated conditions in the flow reactor, a benign solvent such as ethanol may be used at high temperature, and is easily removed by evaporation at the end of the reaction.
|
|
|
|
Note 19: Suzuki-Miyaura
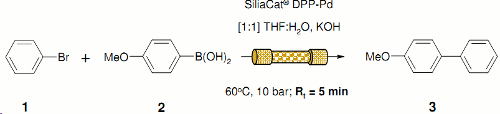
The Suzuki-Miyaura cross-coupling reaction is widely used in synthesis for the preparation of biaryls. However, under homogeneous batch conditions the need to remove the metal catalyst can make product purification difficult. This difficulty can largely be circumvented by operating in a continuous flow-through regime using an immobilised source of palladium.
|
|
|
|
Note 18: Using Pd EnCat for transfer hydrogenation
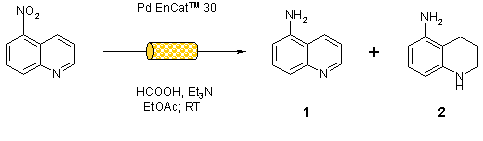
Flow-through conditions afford a high degree of control over reaction parameters. It is frequently possible to perform selective reactions by optimisation of flow conditions. An example follows in which selective reduction of the nitro group can be reproducibly achieved simply by increasing the flow rate.
|
|
|
|
Note 17: Newman Kwart Rearrangement
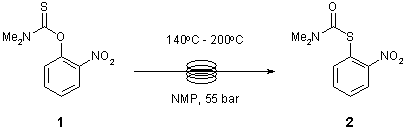
The Newman-Kwart rearrangement is a useful method for converting Ar-O to Ar-S bonds that can be further derivatised following basic hydrolysis of the S-thiocarbamate
|
|
|
|
Note 16 - Fischer Indole Synthesis
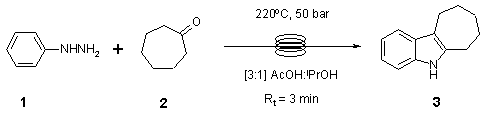
The rate of the Fischer indole synthesis can be significantly increased by running at high temperature and pressure under conditions similar to those encounter in a batch microwave reactor.
|
|
|
|
Note 15: Bohlmann-Rahtz Pyridine Synthesis
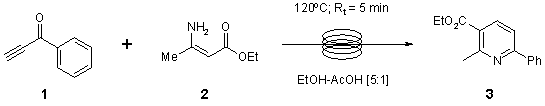
Mark C. Bagley,* Vincenzo Fusillo, M. Caterina Lubinu. School of Chemistry, Main Building, Cardiff University, Park Place, Cardiff, CF10 3AT, UK
|
|
|
|
Note 14: Catch-and-Release Array Synthesis
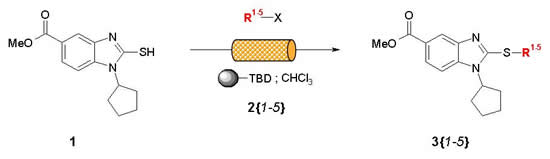
Catch-and-release is a powerful technique that can is well-suited to implementation in flow. In this Application Note a small set of S-alkyl thiobenzimidazolines is prepared as an illustration of this strategy.
|
|
|
|
Note 12: Condensation Reaction
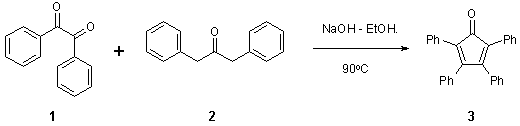
Thomas Wirth* and Johan Brandt, School of Chemistry, Cardiff University, Park Place, Cardiff, CF10 3AT, UK
|
|
|
|
Note 11: Ester Hydrolysis under Segmented Plug Flow Conditions
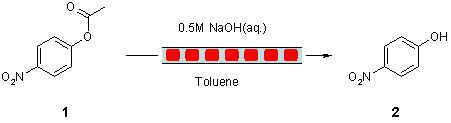
Thomas Wirth*, Johan Brandt, School of Chemistry, Cardiff University, Park Place, Cardiff, CF10 3AT, UK
|
|
|
|
Note 10: Hantzsch Dihydropyridine Synthesis
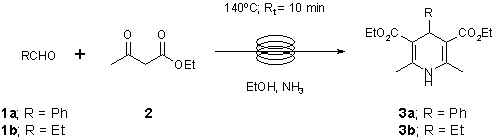
Pressurised continuous flow reactors provide a convenient method for performing reactions with ammonia at elevated temperature. [Mark C. Bagley,* Vincenzo Fusillo, M. Caterina Lubinu. School of Chemistry, Main Building, Cardiff University, Park Place, Cardiff, CF10 3AT, UK]
|
|
|
|
Note 9: Curtius Rearrangement
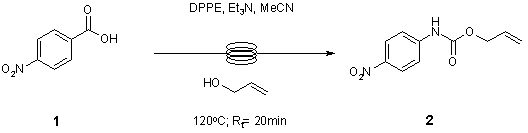
Flow chemistry provides an intrinsically safer procedure for the Curtius rearrangement whereby the quantity of potentially explosive acyl azide intermediate is minimised through continuous processing to the stable final carbamate product.
|
|
|
|
Note 8: Scale-up Synthesis of a Zyvox Intermediate
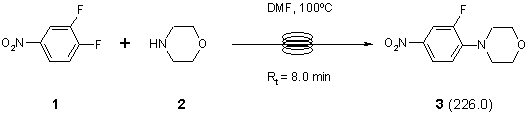
Zyvox™ (Upjohn) was the first example of a new class of oxazolidinone antibiotics that are active against MRSA. In this example, the scale-up abilities of FlowSyn™ are demonstrated by performing the first step in a possible synthesis of this compound on a 100 g scale.
|
|
|
|
Note 7: Chlorothio- benzimidazolinone Synthesis
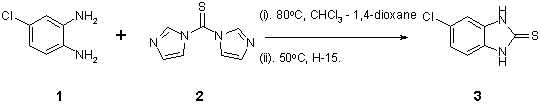
An alternative example to that given in Application Note 6 to afford a thio-heterocycle in high purity following in-line purification
|
|
|
|
Note 6: 2-Thiobenz- imidazolinone Synthesis
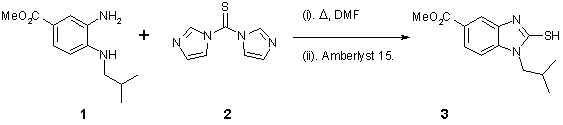
Thiono-acylation followed by an in-line flow through purification is a convenient approach to thiobenzimidazolinones
|
|
|
|
Note 5: Esterification (Example 1)
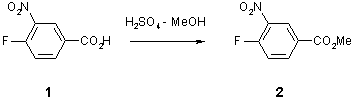
Condensation chemistry can work well under flow-through conditions. Even when, as in this case, there is no provision for removal of the water.
|
|
|
|
Note 4: Esterification
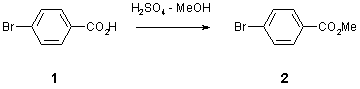
FlowSyn Application Note 4: Esterification
|
|
|
|
Note 3: Knorr Pyrazole Synthesis
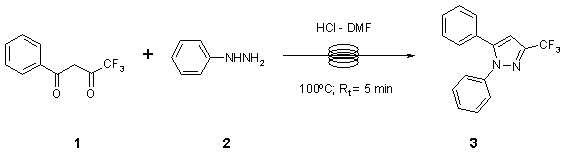
Flow optimization was used to investigate the effects of temperature and residence time. Scale up of these conditions afforded 84% yield of the product with a throughput of 261g/day.
|
|
|
|
Note 2: Ester Hydrolysis
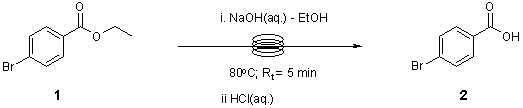
The Ester Hydrolysis reaction was optimized using various temperatures, reaction times and stoichiometry. The reaction was scaled up to provide 92% yield and 105g/day throughput.
|
|
|
|
Note 1: SNAr
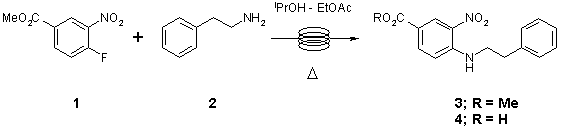
Nucleophilic aromatic substitution works extremely well under continuous flow- through conditions
|